Last Updated: May 2023 (Graphene)
Table of Contents
Graphene
This article deals with ‘Graphene.’ This is part of our series on ‘Science and Technology’ which is an important pillar of the GS-3 syllabus. For more articles, you can click here.
Introduction
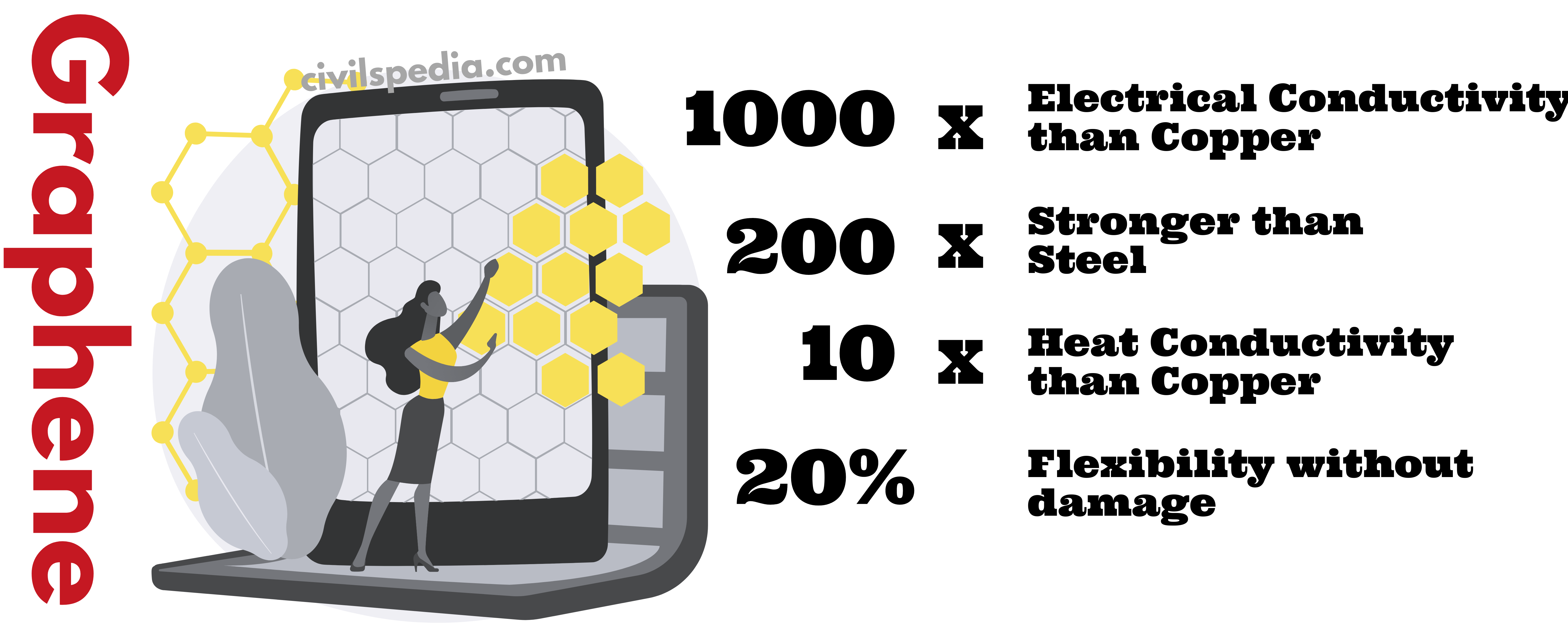
Graphene is a form of carbon consisting of planar sheets (2D structure), which are one atom thick, with the atoms arranged in a honeycomb-shaped lattice.

Properties
- Heat conductivity is 10 times better than Copper
- 200 times stronger than steel
- 1000 times electrical conductivity than Copper wire
- Highly flexible and can flex 20% without damage.
- Transparency is 97% (hence, it can be used to make flexible & unbreakable screens).
- It has a high absorption capacity for Electromagnetic Waves
- It has anti-bacterial properties.
- It is biocompatible, i.e., it can hook up with biological cells.
Applications
- Making Flexible Screens.
- Thermal management applications.
- Solar cells of high efficiency
- To recreate bones as they mimic the environment of the bone.
- Desalination: It can be used as a sheet in the process of reverse osmosis. With Graphene, the energy used in reverse osmosis is 45% less than ordinary process & the process is twice as fast (Note that cost of energy is the most expensive component in the whole process).
But the issue with graphene is it is challenging to make. Presently, large scale studies and experiments are going on to devise a method to make it at a large scale cheaply and out of laboratory conditions.
