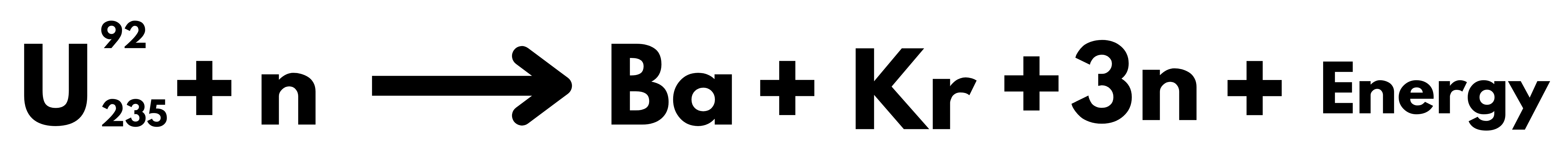Nuclear Fission Reactors
This article deals with ‘Nuclear Fission Reactors .’ This is part of our series on ‘Science and Technology’ which is important pillar of GS-3 syllabus . For more articles , you can click here.
Nuclear Fission
- In 1939, Otto Hahn and Strassman discovered Nuclear Fission when they found that a slow-moving neutron collides with a uranium nucleus; it breaks into two smaller nuclei of comparable masses with the release of energy.
- In simple words, Nuclear Fission means breaking up the heavier nucleus into two smaller nuclei and releasing an enormous amount of energy.
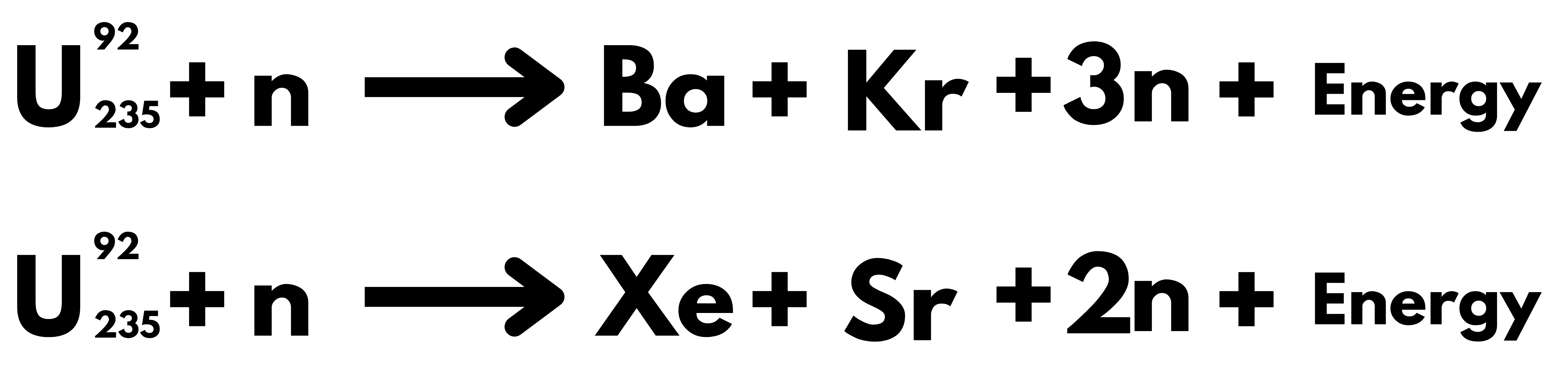
- Reactant total mass is more than product total mass & EXCESS mass is converted to energy (using Einstein’s Mass – Energy Relation (E = mc^2)). The energy released from 1 nucleus of Uranium (235) is nearly 93 Mega Electron Volt. When one Uranium nucleus undergoes fission, the energy released might be small. But from each fission reaction, three neutrons are released. These three neutrons can cause further fission in three other Uranium nuclei. This process is called a chain reaction.
- The energy produced in the nuclear reaction can be used to convert water into steam, which can be converted into electricity using Steam Turbine and Generator.
Types of Reactors
A simple Nuclear Reactor from which electricity can be generated is of following type
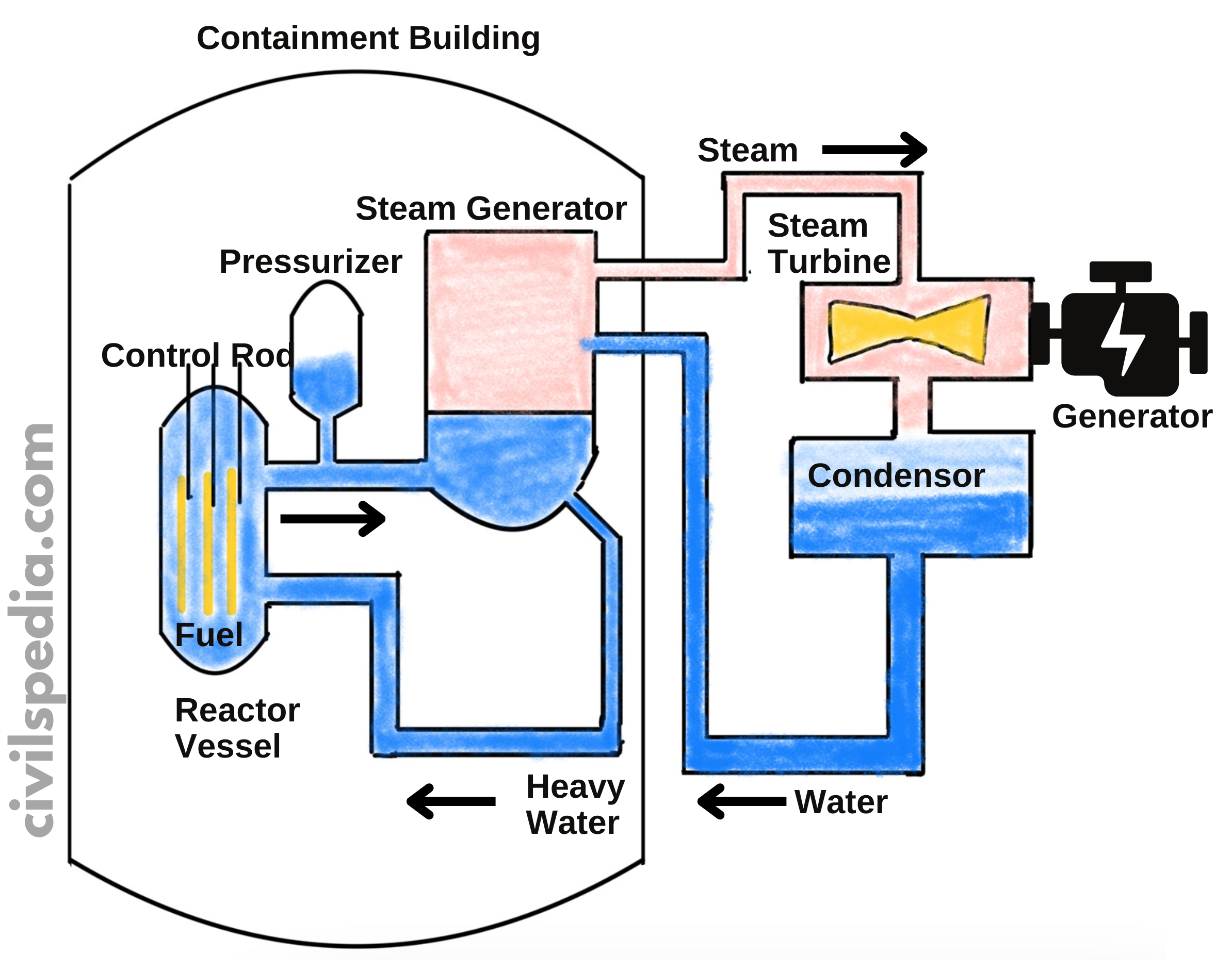
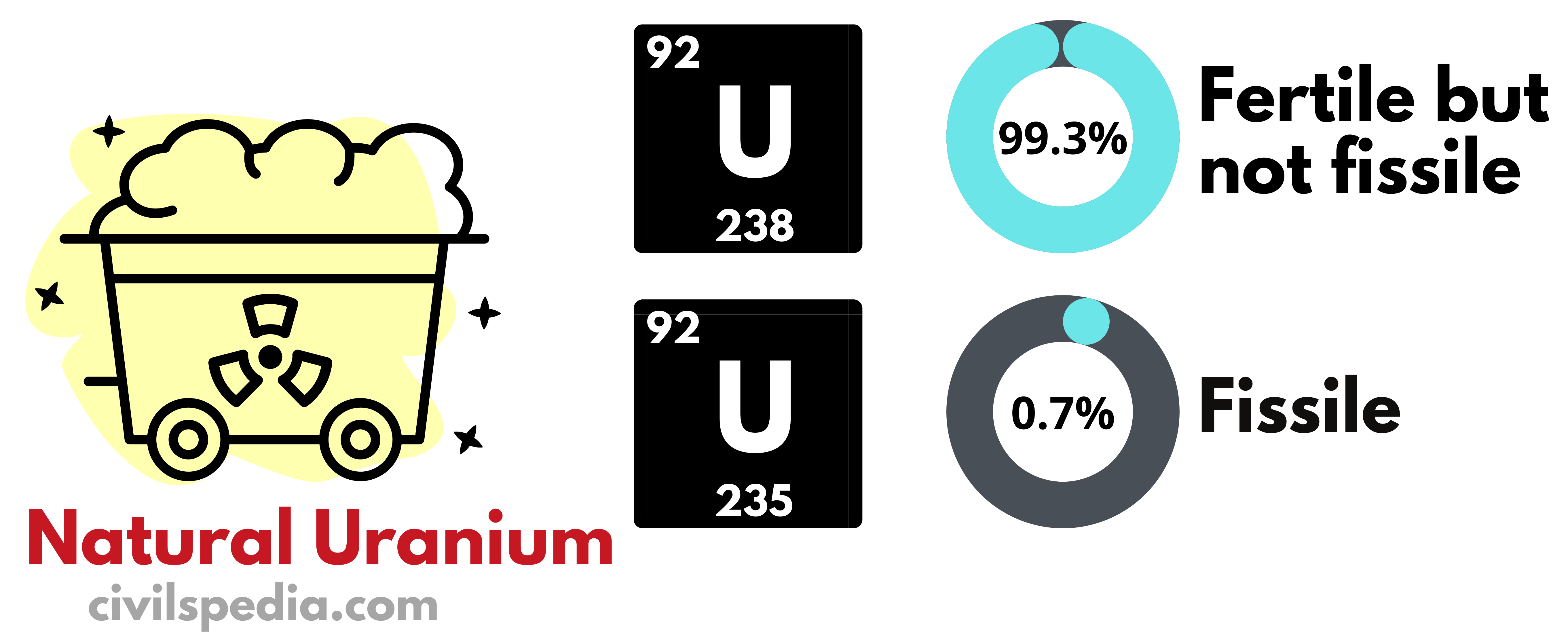
| Fuel | U-235, U-233, Pu – 239 or Th -232 is used as fuel in the Nuclear Reactor |
| Moderator | – It reduces the speed of neutrons so that nuclear reactions can take place. – Graphite or Heavy Water (D2O) is used as Moderator. |
| Coolant | – The coolant absorbs the energy/heat released from the reaction and transfers it into turbines. – Heavy Water or Water can be used as coolant (depending on the type of Reactor) |
| Control Rods | – To control the speed of the Nuclear Reactor. – Boron or Cadmium is used as Control Rod. |
| Concrete Shield | Concrete wall with 2-5 m thickness to stop radiation from spreading. |
Reactors used in India
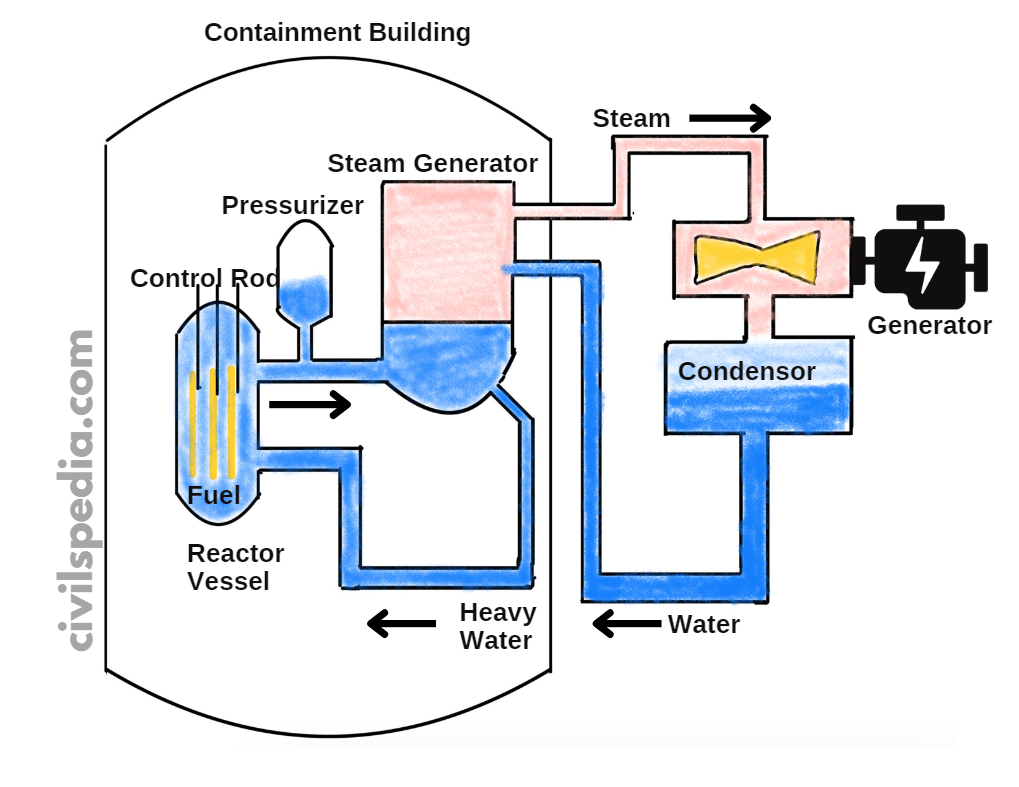
1. Pressurized Heavy Water Reactor (PHWR)
Most of the Nuclear Reactors found in India are PHWRs.
Information at Glance
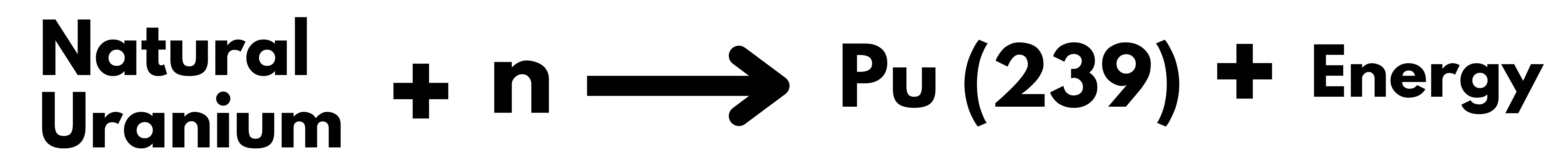
| Fuel | Natural Uranium (without enrichment) (It is easier to make and less expensive to use, as Uranium enrichment is a complex process) |
| Coolant | Heavy Water/Deuterium Oxide |
| By Products | Plutonium (more amount ) |
| Moderator | Heavy Water/Deuterium Oxide (Moderator and coolant are same) => Neutrons collide with Heavy Water molecules and slow them. |
| Why Pressurized | If water is heated, it expands & becomes less dense. As a result probability of collision between neutrons and water molecules to reduce the speed of neutrons decreases. It is crucial to decrease the speed of neutrons to ensure fission. |
| Cost | Less Expensive |
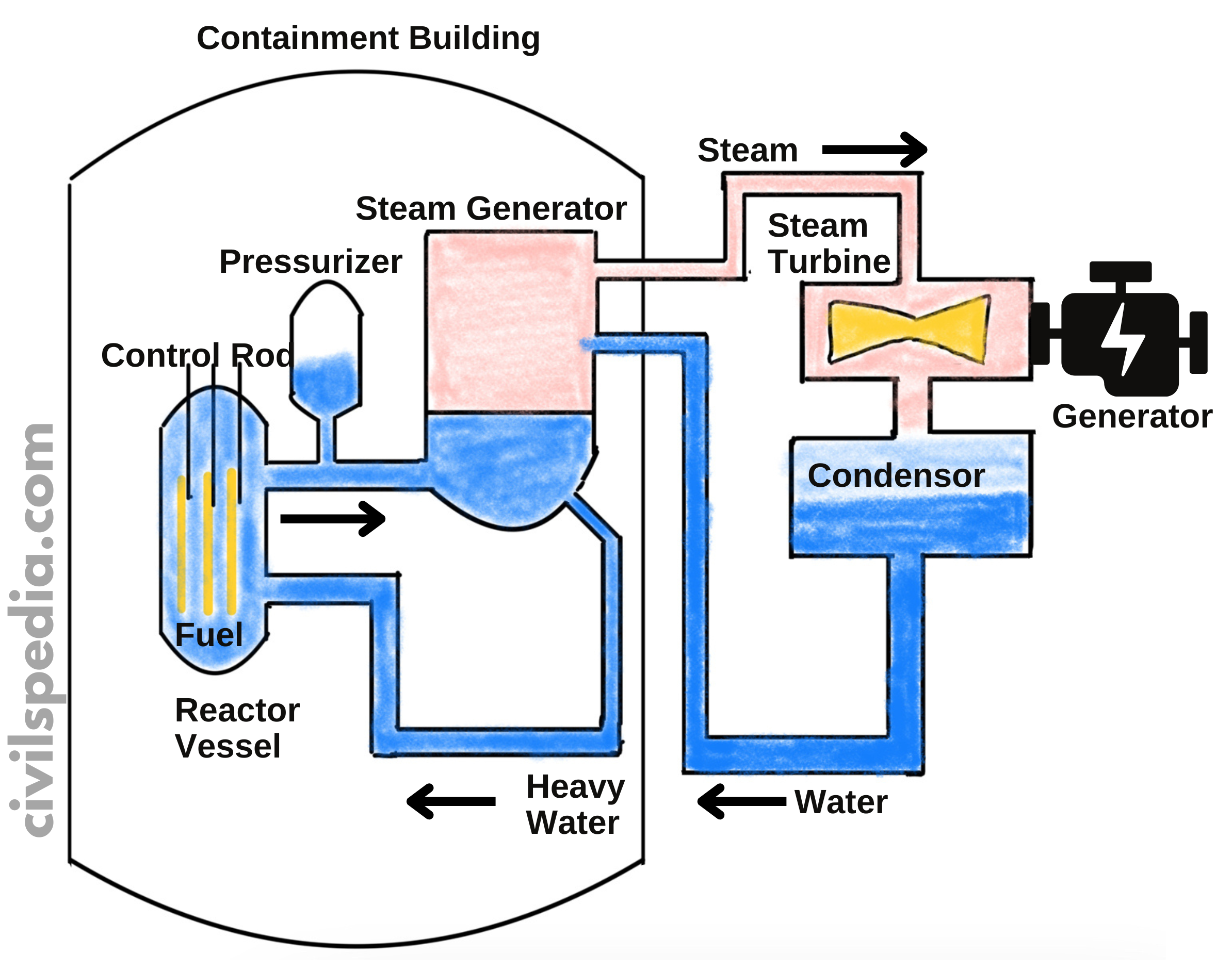
Details of PHWRs
The primary reaction which leads to the generation of energy while using Uranium as fuel is
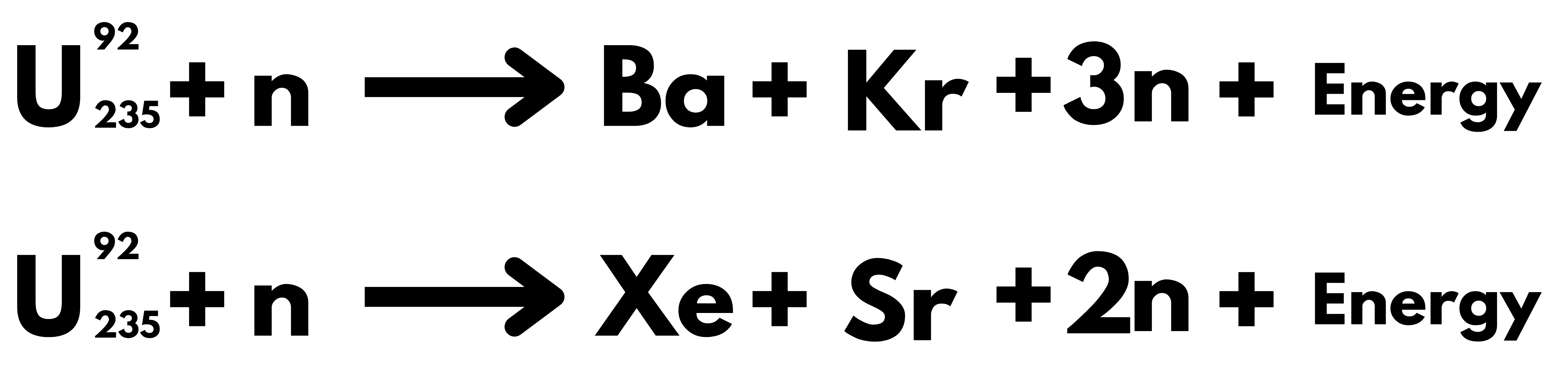
- The most crucial point in Uranium Fission Reaction is the release of an extra 2.5 (average) Neutrons, which leads to the possibility of a chain reaction. If controlled, it can be used to produce energy called Nuclear Energy. At the same time, if it remains uncontrolled, it can result in an Atomic Bomb.
- But the biggest hurdle, in this case, is the fact that neutrons liberated in the nuclear reaction are fast-moving & will not cause fission (instead, they will escape without causing any collision). To ensure a Fission reaction, these neutrons must be slowed. For this purpose, Moderators are used, which in this case are D2O (Heavy Water) & Graphite.
- The reaction rate can be controlled by Control Rods, which are made up of neutron-absorbing material like Cadmium.
- The energy released in fission is continuously removed by a suitable Coolant which transfers heat to a working fluid which in turn may produce steam to drive the turbine & generate electricity.
- Pressuriser is used because when heavy water is heated, it expands & becomes less dense. As a result probability of collision between neutrons and heavy water molecules to reduce the speed of neutrons decreases. It is crucial to decrease the speed of neutrons to ensure fission. The Pressuriser ensures the suitable density of the heavy water.
2. Boiling Water Reactor (BWR)
- It is the oldest type of Nuclear Reactor.
- Fuel Used: Enriched Uranium
- Working: Energy released during the fission reaction directly heats the (light) water. The same water is used to turn the turbine and then recycled back, to be used again in the cycle.
- Moderator: No Moderator is used. The probability of neutron colliding with U-235 is achieved by using Enriched Uranium.
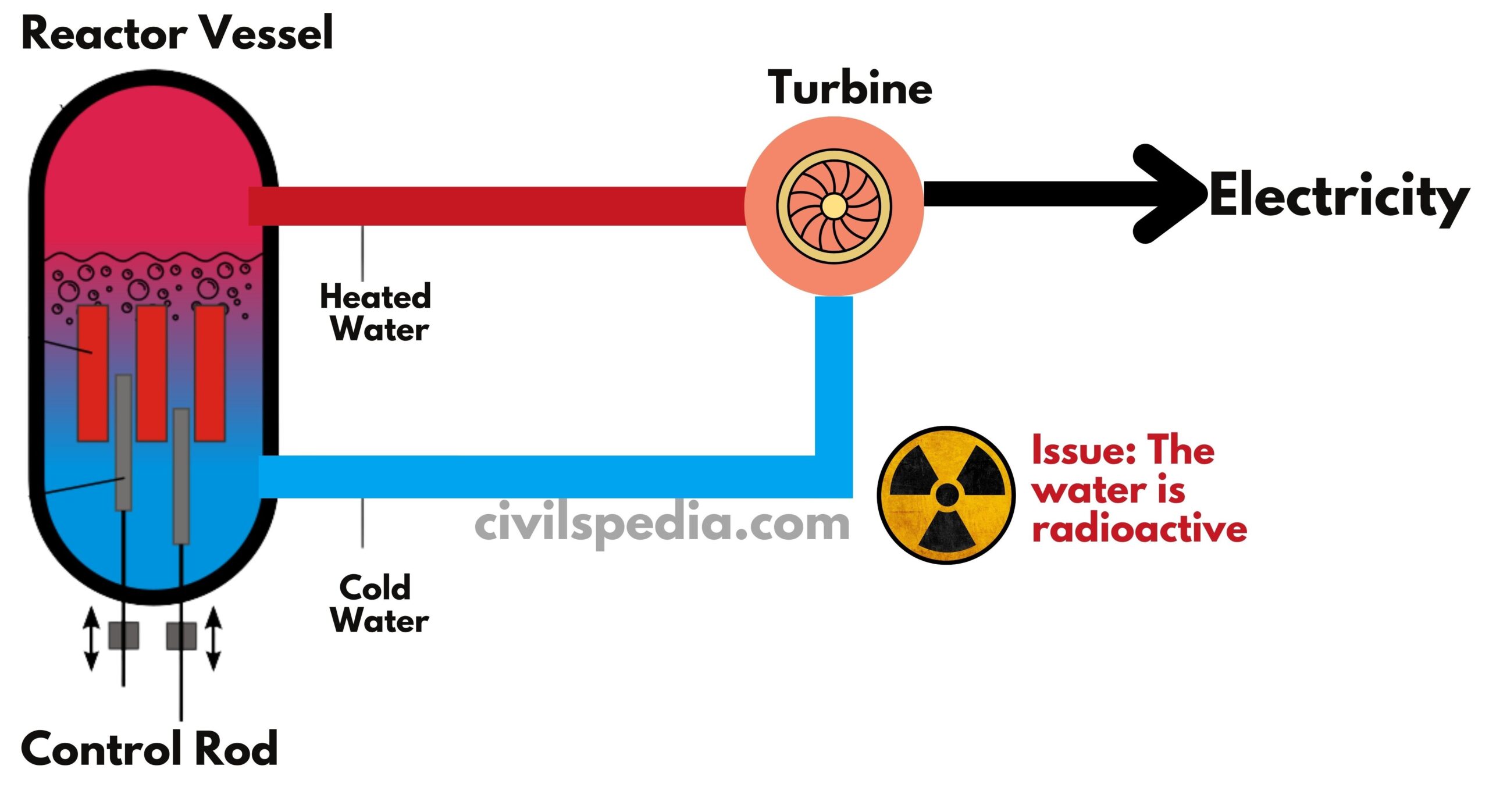
- BWRs are the second most widely used reactors in the world. But in India, we don’t use them on a large scale. Tarapur Atomic Power Station was constructed initially with two boiling water reactors (BWR) under the 1963 Agreement between India, USA & International Atomic Energy Agency (IAEA).
Issue with BWR
- The use of enriched Uranium increases cost and complexities.
- Light water is directly heated by radioactive material. Hence, nuclear radiation fallout in case of an accident is maximum in such reactors. For example, Japan’s Fukushima Nuclear reactor, which caused great damage after a nuclear accident, was BWR.
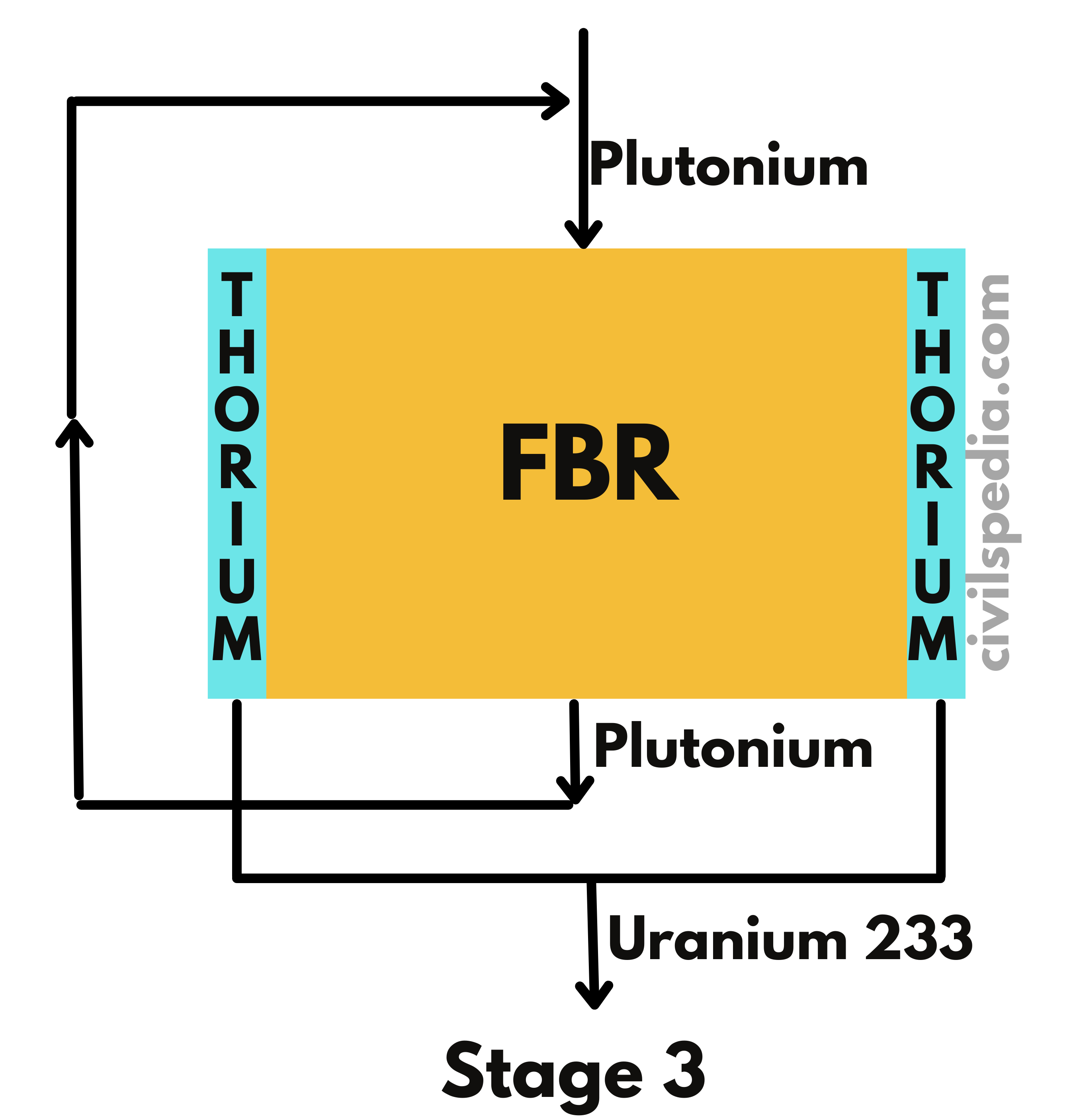
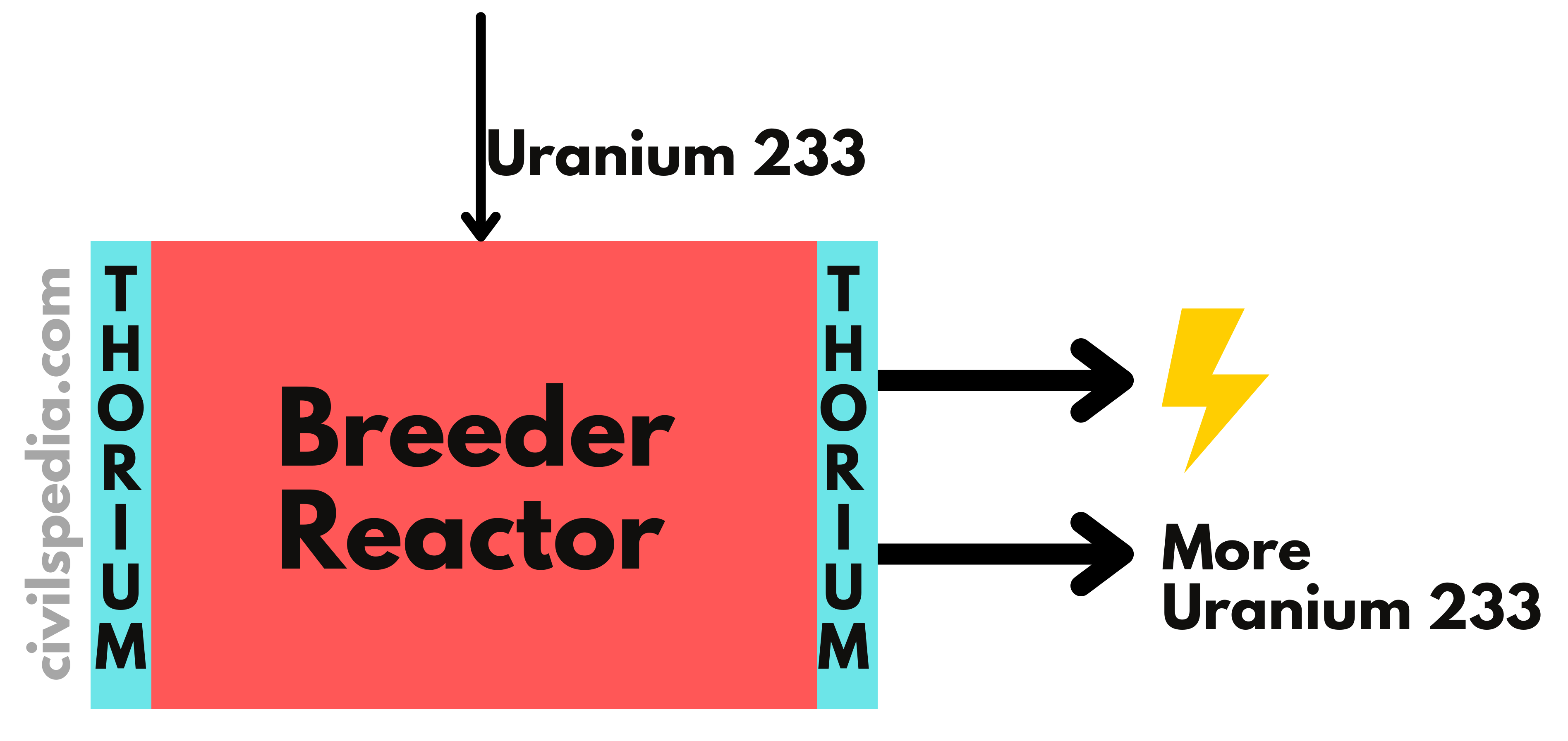
3. Fast Breeder Reactor
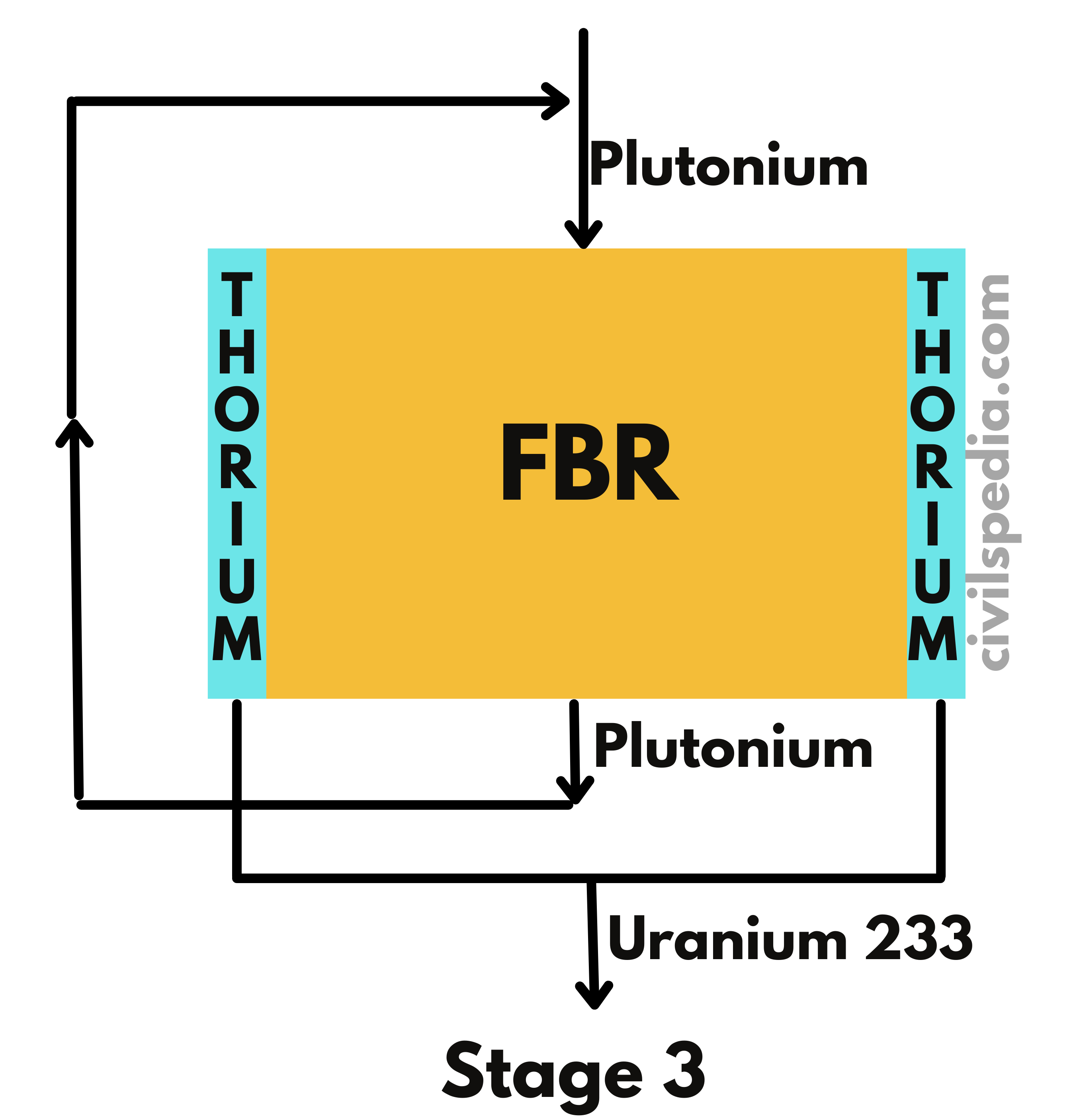
- Fast Breeder Reactor produces the same kind of fissile material as it burns.
- While using Pu239 as fuel, it can produce more Pu239 than it consumes by converting non-fissionable U-238 present in the natural Uranium.
- With fast neutrons, the chances of absorption by U-238 increase. Additionally, Pu-239 produces extra neutrons in the case of a collision with fast-moving neutrons only. Hence, these reactors don’t use moderators to slow down the neutrons.
- Liquid sodium or steam coolants are used in FBRs.
- India’s BHAVINI nuclear reactor is Prototype Fast Breeder Reactor.
Prelims Related: List of BARC Atomic Reactors
| Apsara | – First Atomic Reactor in 1957 |
| Cirus | – Indo-Canadian Reactor – Operational Period: 1960-2010 |
| Zerlina | – Operationalized in 1961 – To study Uranium Heavy Water Reactors |
| Dhruva | – Operationalized in 1984 – Completely indigenous reactor |
| Purnima -1 | |
| Kamini | – India’s first Fast Breeder Reactor. – Installed in Kalpakkam – India is 7th country in world to have FBR |